Histotripsy uses nano- or micrometer-sized gas pockets inside the tissue as cavitation nuclei to generate a cloud of microbubbles (i.e., cavitation cloud) [1,2]. Typically microsecond-length pulses (1-20 cycles) at a center frequency of 200kHz – 5MHz and very high peak negative pressure in situ [3-5] are used. There are two approaches to generate a cavitation cloud: 1) intrinsic threshold approach [1] and shock scattering approach [6]. Using the intrinsic threshold approach, a cavitation cloud can be reliably generated using a single 1-2 cycle ultrasound pulse when the peak negative pressure directly exceeds the cavitation threshold intrinsic to the tissue. This threshold was measured to be from –26 to –28 MPa in water-based tissue, including blood clots, kidney, liver, heart, spleen, etc., and to be –14 MPa in lipid-based tissue [1]. If the peak negative pressure is right above the intrinsic threshold, this cloud of cavitation microbubbles can be smaller than the focal zone. Thus, the intrinsic threshold histotripsy is also termed microtripsy by its capability of generating sub-millimeter cavitation clouds smaller than the diffraction limit defined by the ultrasound wavelength [5].
When the peak negative pressure is below the intrinsic threshold, a cavitation cloud can be generated via shock scattering histotripsy [6]. Using a multi-cycle ultrasound pulse (typically 3-20 cycles), an individual microbubble can be generated in the first 1-3 cycles with the peak negative pressure of about -20 MPa in the water-based tissue, if there is a pre-existing cavitation nucleus (e.g., 100nm gas pocket) in the focal zone. At high pressures used in histotripsy, due to non-linear acoustic propagation, the shock front develops in each cycle of the ultrasound waveform, and the peak positive pressure in the waveform is much higher than the peak negative pressure (e.g., can be p+>80MPa corresponding to |p-| = 20MPa). The high-amplitude shock in the subsequent acoustic cycle of a multi-cycle ultrasound pulse scatters back from the soft boundary of the initial individual microbubble and forms an inverted shock a high negative pressure. This inverted shock and the incoming negative phase in the subsequent acoustic cycle together result in extremely high negative pressure, well beyond the intrinsic threshold, and generate a cloud of dense microbubbles.
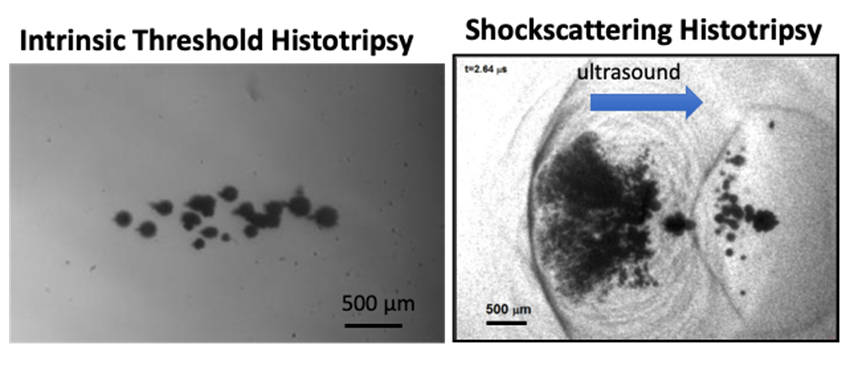
Figure: High speed optical images of the cavitation bubble cloud generated by intrinsic threshold histotripsy (left) and shock scattering histotripsy (right).
Both intrinsic threshold and shock scattering histotripsy use a very low duty cycle (ultrasound on-time/total treatment time = 0.0001% – 1%) to mitigate heating. After cavitation nucleation, the nanometer-sized gas pockets can grow to hundreds of microns and collapse within a few hundred microseconds. The rapid expansion of the cavitation bubbles squeezes the adjacent cells, the bubble collapse pulls the adjacent cells, and this local high strain and stress produced over multiple pulses results in cyclic strain and stress imposing on the cells and eventually mechanically breaking them down [7]. Increasing the number of pulses results in increasing extent of cellular and subcellular disruption within the focal zone. Eventually, the tissue within the focal zone can be completely liquefied into acellular debris with no intact cells remaining. The boundary zone between complete cell disruption and surrounding intact cells is very narrow within 1mm in vivo and 100µm in vitro.
References
[1] Maxwell, A. D., Cain, C. A., Hall, T. L., Fowlkes, J. B. & Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 39, 449-465, (2013). 3570716
[2] Bader, K. B., Vlaisavljevich, E. & Maxwell, A. D. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med Biol 45, 1056-1080, (2019). PMC6524960
[3] Xu, Z., Ludomirsky, A., Eun, L. Y., Hall, T. L., Tran, B. C., Fowlkes, J. B. & Cain, C. A. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51, 726-736, (2004). PMC2669757
[4] Parsons, J. E., Cain, C. A., Abrams, G. D. & Fowlkes, J. B. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med. Biol. 32, 115-129, (2006).
[5] Lin, K. W., Kim, Y., Maxwell, A. D., Wang, T. Y., Hall, T. L., Xu, Z., Fowlkes, J. B. & Cain, C. A. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 251- 265, (2014). 3966303
[6] Maxwell, A. D., Wang, T. Y., Cain, C. A., Fowlkes, J. B., Sapozhnikov, O. A., Bailey, M. R. & Xu, Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. The Journal of the Acoustical Society of America 130, 1888-1898, (2011). 3206907
[7] Vlaisavljevich, E., Maxwell, A., Mancia, L., Johnsen, E., Cain, C. & Xu, Z. Visualizing the Histotripsy Process: Bubble Cloud-Cancer Cell Interactions in a Tissue-Mimicking Environment. Ultrasound Med Biol 42, 2466-2477, (2016). PMC5010997
