Transcranial histotripsy uses microtripsy (1-cycle pulses) ultrasound pulses through the skull to generate highly precise focal cavitation and liquefy the target tissue into acellular debris [1,2]. When the peak negative pressure exceeds the cavitation intrinsic threshold (26 MPa in the brain) [3], a millimeter sized cloud of cavitation microbubbles is generated from endogenous nanometer gas pockets. The rapid expansion and collapse of cavitation microbubbles induces high strain to mechanically break down cells in the target region [1] into liquid homogenate. Intrinsic threshold histotripsy or microtripsy using 1-cycle pulses [4] is used to ensure the highest treatment accuracy (1-2mm) in the brain [2]. Using a very low duty cycle (<0.1%) to minimize the skull heating, transcranial histotripsy has the potential to treat various brain pathologies in a wide range of locations in the brain and volumes without overheating the skull [5], in comparison to the limited treatment location and volume envelop of transcranial MR-guided focused ultrasound (tcMRgFUS) thermal ablation.
Transcranial Histotripsy Transducers
We have designed and built four transcranial histotripsy phased array transducers, all of which are hemispherical shaped with a 30-cm diameter. Two are 256-element arrays, with a center frequency of 250kHz and 500kHz respectively [5]. The other two arrays are both 700kHz, one is a 360-element array guided by neuronavigation system, and the other is an MRI-compatible 128-element array (a truncated version of the 360-element array) designed for pig experiments [6] (Fig. 1). All four transducer can produce 1-cycle pulses with >30MPa peak negative pressure through an excised human skull for microtripsy. The 700kHz, 360-element produces the highest pressure and the highest treatment accuracy among these four arrays.
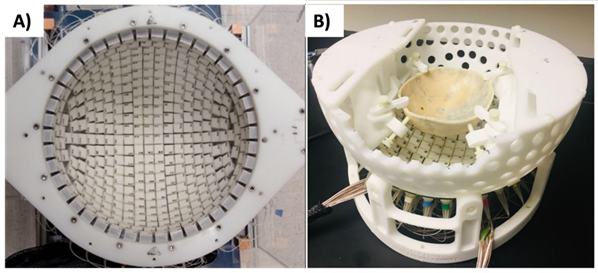
Fig. 1: A) The 700kHz, 360-element transcranial histotripsy array. B) The 700kHz, 128-element, MR-compatible transcranial histotripsy array.
In vivo Pig Brain Treated through an Excised Human Skull
To determine the feasibility of in vivo transcranial histotripsy, transcranial MR-guided histotripsy (tcMRgHt) treatment was delivered to eight pigs using a 700-kHz, 128-element, MR-compatible phased-array transducer inside a 3-T magnetic resonance imaging (MRI) scanner. After craniotomy to open an acoustic window to the brain, histotripsy was applied through an excised human skullcap to target the inside of the pig brain based on pre-treatment MRI and fiducial markers. Successful histotripsy ablation was observed in all pigs. The MR-evident lesions were well confined within the targeted volume, without evidence of excessive brain edema or hemorrhage outside of the target zone. Histology revealed tissue homogenization in the ablation zones with a sharp demarcation between destroyed and unaffected tissue, which correlated well with the radiographic treatment zones on MRI (Fig. 2). These results are the first to support the in vivo feasibility of tcMRgHt in the pig brain, enabling further investigation of the use of tcMRgHt for brain surgery.
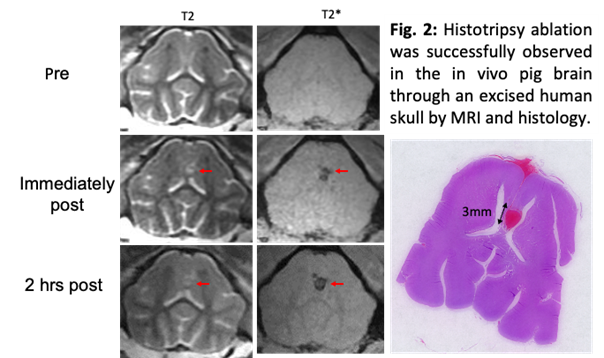
Intracerebral Hemorrhage
Intracerebral hemorrhage (ICH) is a devastating form of stroke, leading to a 30-day survival rate of 40-50% and significant disability for those that survive. ICH is characterized by the rupture of vessels resulting in bleeding and clotting inside the brain. Histotripsy has been used to quickly liquefy the clot through the skull, and the liquefied clot can be drained via catheter inserted through a small hole in the skull. This allows removal of liquefied clot with minimal collateral damage. With the development of a catheter hydrophone [7] and guided by a neuronavigation system and pretreatment MRI/CT brain scans, transcranial histotripsy can be used to treat ICH patients outside the MRI scanner.This allows the potential for histotripsy to be applied in a standalone system and thus increases the potential for adoption of histotripsy into widespread clinical use.
We have demonstrated volume liquefaction of clots through excised human skulls using electronic focal steering with our 250kHz and 500kHz 256-element hemisphere arrays using 1-cycle pulses at peak negative pressure > 30MPa, and 200 Hz pulse repetition frequency (PRF) (corresponding to 0.04% and 0.08% duty cycle). Clot liquefaction was achieved and drained with a catheter in a range of 4.1 – 54.1 mL in 0.9 – 42.4 minutes, resulting in liquefaction rates of 0.5 – 12.6 mL/min [5]. Monitoring the temperature increase within the skull during these in-vitro experiments showed a temperature rise that remained below 4 °C, which is reported as significantly below the threshold required to cause damage bone or the surrounding tissue.
To test the safety of histotripsy treatment of ICH in-vivo, we used a well-accepted porcine ICH model [8]. The focus of a histotripsy transducer was mechanically scanned through the clot to liquefy the central portion of the clot. A small amount of intact clot was left around the perimeter of the clot to avoid damaging the brain. MR images histology slides of the treated brains show that the histotripsy-liquefied volume was confined inside the clot, leaving 0.5 – 1mm rim of clot untreated purposely (Fig. 3). No damage or hemorrhage to the surrounding brain tissue was observed.
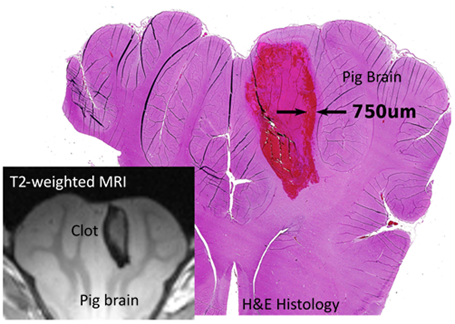
Fig. 3: Histology and MRI shows that a clot in the in vivo pig brain was liquefied by histotripsy, leaving 0.5 – 1mm rim of clot untreated purposely.
Murine Brain Tumor Model
The effect of various histotripsy dosages on tumor cell kill and associated bleeding was investigated in two murine brain tumor models (glioma [Gl261] and lung metastasis [LL/2-Luc2]) [9]. GL261 or LL/2-Luc2 cells were cultured and implanted into the brains of C57BL/6 mice. Transcranial histotripsy (1-cycle pulses, 5 Hz PRF, 30 MPa-P) was performed using a 1 MHz transducer for five different dosages for each cell line: 5, 20 or 200 pulses per location (PPL) at a single treatment point, or 5 or 10-20 PPL at multiple treatment points. MRI, bioluminescence imaging and histology were used to assess tumor ablation and treatment effects within 4-6 h post-treatment. All treatment groups resulted in a reduction of BLI intensity for the LL/2-Luc2 tumors, with significant signal reductions for the multi-point groups. The average pre-/post-treatment BLI flux (photons/s, x10(8)) for the different treatment groups were: 4.39/2.19 (5 PPL single-point), 5.49/1.80 (20 PPL single-point), 3.86/1.73 (200 PPL single-point), 2.44/1.11 (5 PPL multi-point) and 5.85/0.80 (10 PPL multi-point). MRI and H&E staining showed increased tumor damage and hemorrhagic effects with increasing histotripsy dose for both GL261 and LL/2-Luc2 tumors, but the increase in tumor damage was diminished beyond 10-20 PPL for single-point treatments and outweighed by increased hemorrhage. In general, hemorrhage was confined to be within 1 mm of the treatment boundary for all groups. Our results suggest that a lower number of histotripsy pulses at fewer focal locations can achieve substantial tumor kill while minimizing hemorrhage.
References
[1] Vlaisavljevich, E., Maxwell, A., Mancia, L., Johnsen, E., Cain, C. & Xu, Z. Visualizing the Histotripsy Process: Bubble Cloud-Cancer Cell Interactions in a Tissue-Mimicking Environment. Ultrasound Med Biol 42, 2466-2477, (2016). PMC5010997
[2] Lu, N., Gupta, D., Daou, B. J., Fox, A., Choi, D., Sukovich, J. R., Hall, T. L., Camelo- Piragua, S., Chaudhary, N., Snell, J., Pandey, A. S., Noll, D. C. & Xu, Z. Transcranial Magnetic Resonance-Guided Histotripsy for Brain Surgery: Pre-clinical Investigation. Ultrasound Med Biol 48, 98-110, (2022).
[3] Maxwell, A. D., Cain, C. A., Hall, T. L., Fowlkes, J. B. & Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 39, 449-465, (2013). 3570716
[4] Lin, K. W., Kim, Y., Maxwell, A. D., Wang, T. Y., Hall, T. L., Xu, Z., Fowlkes, J. B. & Cain, C. A. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 251- 265, (2014). 3966303
[5] Gerhardson, T., Sukovich, J. R., Pandey, A. S., Hall, T. L., Cain, C. A. & Xu, Z. Effect of Frequency and Focal Spacing on Transcranial Histotripsy Clot Liquefaction, Using Electronic Focal Steering. Ultrasound Med Biol 43, 2302-2317, (2017). PMC5580808
[6] Lu, N., Hall, T. L., Choi, D., Gupta, D., Daou, B. J., Sukovich, J. R., Fox, A., Gerhardson, T. I., Pandey, A. S., Noll, D. C. & Xu, Z. Transcranial MR-Guided Histotripsy System. IEEE Trans Ultrason Ferroelectr Freq Control 68, 2917-2929, (2021). PMC8428576
[7] Gerhardson, T., Sukovich, J. R., Pandey, A. S., Hall, T. L., Cain, C. A. & Xu, Z. Catheter Hydrophone Aberration Correction for Transcranial Histotripsy Treatment of Intracerebral Hemorrhage: Proof-of-Concept. IEEE Trans Ultrason Ferroelectr Freq Control 64, 1684-1697, (2017). PMC5681355
[8] Gerhardson, T., Sukovich, J. R., Chaudhary, N., Chenevert, T. L., Ives, K., Hall, T. L., Camelo-Piragua, S., Xu, Z. & Pandey, A. S. Histotripsy Clot Liquefaction in a Porcine Intracerebral Hemorrhage Model. Neurosurgery 86, 429-436, (2020). PMC7308653
[9] Duclos, S., Golin, A., Fox, A., Chaudhary, N., Camelo-Piragua, S., Pandey, A. & Xu, Z. Transcranial histotripsy parameter study in primary and metastatic murine brain tumor models. Int. J. Hyperthermia 40, 2237218, (2023).
