Our group has studied histotripsy for treatment of liver cancer [1,2], prostate cancer [3-5], renal cancer [6,7], melanoma [8], pancreatic cancer as well as immune response stimulation [8,9]. Our research has led to a Phase I trial on benign prostatic hyperplasia treatment (NCT01896973)[10], a Phase I trial on liver cancer treatment (NCT03741088) [11,12], and multi-center clinical trials on liver cancer treatment in the U.S. (NCT04572633) and Europe (NCT04573881), all using HistoSonics devices. Here we provide a summary of our histotripsy small animal and large animal research on liver cancer treatment, the initial results on human trial, and the immunostimulation results.
Liver Cancer
Rodent liver tumor model – Our study in an orthotopic liver tumor model showed lengthened survival following partial histotripsy ablation of the liver tumor [1]. Rats were inoculated with McA-RH7777 cells. All 11 untreated control animals developed intrahepatic metastasis and had to be euthanized within 3 weeks (Fig. 1A). In 11 treatment rats, at 7-9 days post McA-RH7777 tumor inoculation, when the tumor grew to 5-10 mm and right before metastasis started to form, ~50-75% of the primary tumor volume was treated by ultrasound-guided histotripsy using 1 cycle pulses at 100 Hz PRF (p- >30 MPa) and 300–600 pulses/mm3 delivered by a 1 MHz transducer. Complete local tumor regression was observed on MRI in 9/11 treated rats with no local recurrence or metastasis up to the 12-week study endpoint post histotripsy (Fig. 1B), and only a <1mm residual scar tissue observed on histology. Histology of the untreated control tumor at day 7 after the treatment time point showed invasive appearance at tumor periphery and multiple secondary tumor nodules. In comparison, at day 7 post histotripsy, there was fibrotic or scar tissue beginning to form, no intact tumor cells, and enhanced immune cell and Inflammatory cell infiltration, as compared to controls. Survival outcomes in histotripsy-treated animals (mean ± SEM; 10 ± 0.84 weeks post treatment) were significantly improved compared to controls (1.45 ± 0.69 weeks) (p-value < 0.0001). In a small cohort of animals (n=2), multicentric tumors were generated in separate liver lobes and only one of the tumors was entirely targeted by histotripsy. Abscopal effects were observed in the separate, untargeted tumor, and both tumor nodules demonstrated complete regression.
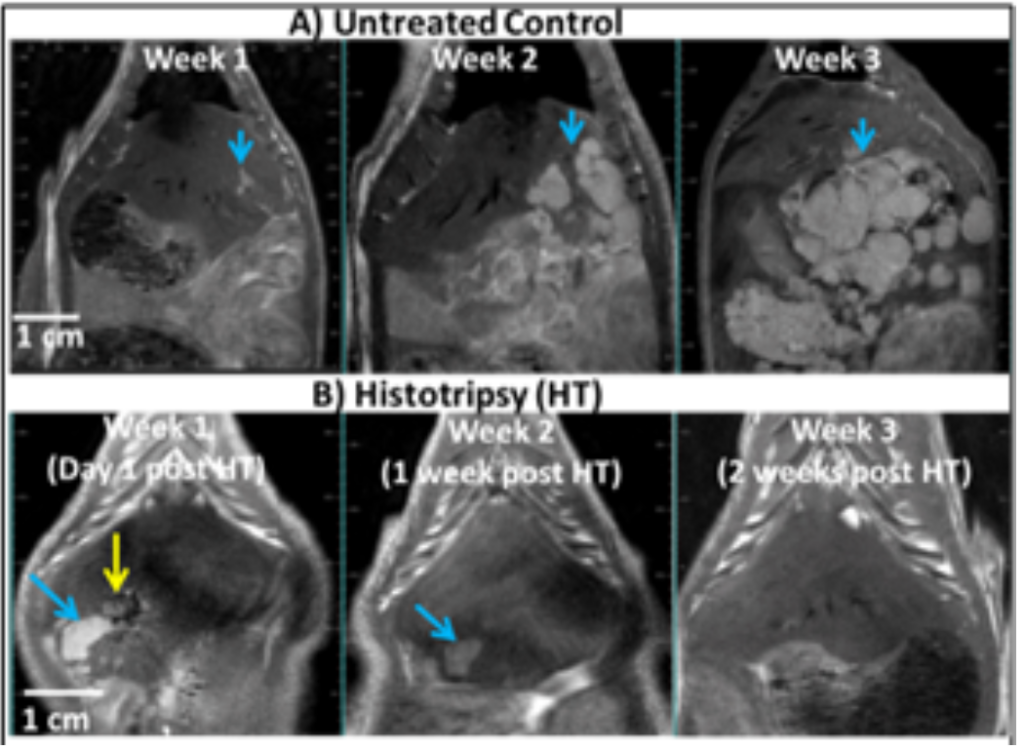
Fig. 1: A) T1-weighted MR images show McA-RH7777 tumors (hyperintense, blue arrows) in the liver of an untreated rat. The intrahepatic metastasis grew aggressively by 2-3 weeks. B) MR images show one tumor.
Porcine liver tumor model – Histotripsy has been shown to create effective and safe ablations in the in vivo human-scale porcine normal liver and the following results (Fig. 2): (1) consistent cellular disruption in the target through intact ribs and other tissue without damage along the energy delivery path; (2) sharp margins <1 mm without significant inflammation; (3) capability to ablate tumors of any shape, size, and nodule number; (4) complete ablation of a 60 mL liver volume in 60 minutes (at a speed of 1mL/min) with decreasing ablation times expected after parameter optimization.
A target liver volume of 12-60 mL through 3-12 cm of overlying tissue was completely ablated in 20-75 minutes [13,14] using 5-cycle pulses at 750 kHz with 200Hz PRF and peak negative pressure >20MPa. Within the ablated region, uniform tissue disruption was achieved with no intact cells remaining, while major vessels and bile ducts were intact due to the tissue-selectivity of histotripsy.
As bones are highly reflective and absorptive for ultrasound propagation, the feasibility and safety of histotripsy through ribs was studied. A focal pressure just above the cavitation threshold was used by gradually increasing the ultrasound power until cavitation was observed on ultrasound. The ablation zones created through full ribcage coverage vs. only overlying soft tissue had comparable dimensions. The highest temperature increase to the ribs with full ribcage covering the pathway was 4.1 ± 1.8 ºC [15], which was not sufficient to cause thermal damage to the ribs or surrounding tissue. No body wall damage was observed on MRI.
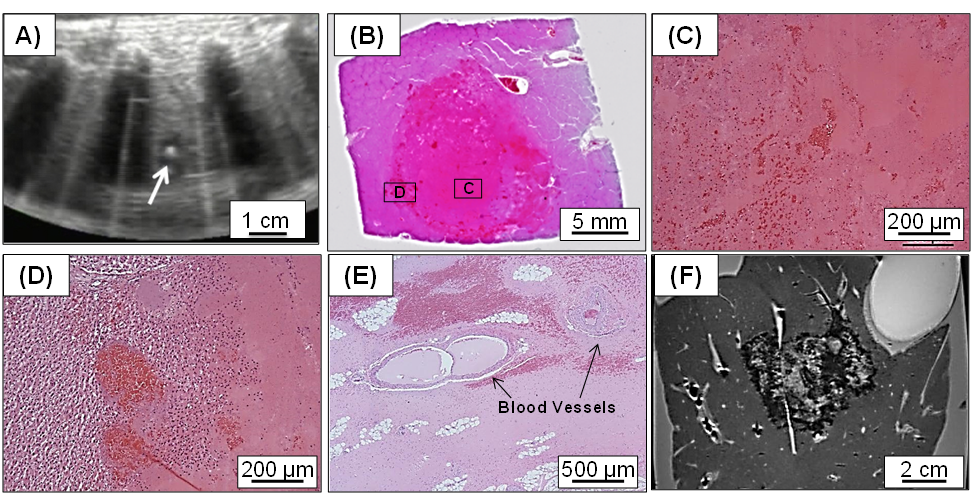
Fig. 2: In vivo histotripsy liver ablation in the porcine liver. A) Focal cavitation generated by histotripsy shows as the bright spot on ultrasound image during treatment. B) Histology of the histotripsy ablation zone, with the magnified view shows no intact cells within the ablation zone (C) and a sharp margin (D). E) Intact vessels >300 um remain within the ablation zone, as vessels and bile ducts are more resistant to histotripsy-induced damage. E) MR image of a 60 mL liver volume (equivalent to ~5 cm diameter) was treated within an hour by mechanically moving the histotripsy focus to cover the target volume.
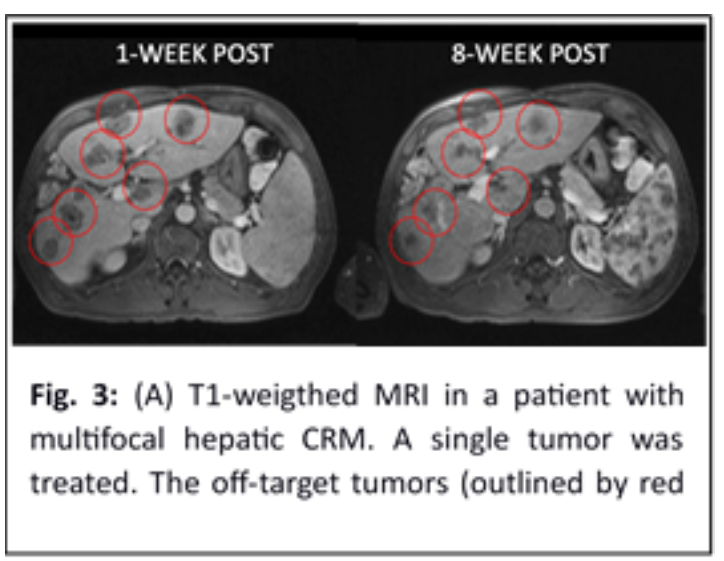
Phase I human trial – The first human trial of hepatic histotripsy was conducted in 8 patients with incurable multifocal liver malignancy in Spain in 2019 (NCT03741088) [11]. A prototype histotripsy device built by HistoSonics was used. Eleven tumors (range 0.5 to 2.1 cm) were targeted by US imaging and treated within 30 minutes. A single 5 mm tumor was mistargeted due to invisibility by US, while the other 10 tumors were successfully ablated, and local tumor regression was evident by MRI, showing volume contraction of the treated tumor averaging 36.0% at 1 week, 53.6% at 1 month, and 71.8% at 2 months. No procedure-related significant adverse events occurred. Two of the 8 patients, one each with multifocal HCC and multifocal colorectal malignancy (CRM), had a continuous decline in tumor markers post histotripsy with non-target tumors significantly decreased on MRI at 8 weeks in the patient with CRM [12] (Fig. 3). This provides the first evidence of abscopal effect and metastasis reduction induced by histotripsy in humans.
Immune Response Stimulation
Histotripsy has been shown to stimulate an innate and adaptive potent immune response in murine melanoma and liver tumor models [8,9]. This was manifested by an increase in CD8+ TIL cells, Neutrophil (Ly6G+ cells), tumor-specific T cells, Dendritic cells (CD11c+), high-mobility group box 1 (HMGB1), and activated CD8+ T cells (as indicated by intracellular interferon gamma (IFNγ) expression)) at 7-10 days post-treatment. Histotripsy also induced a strong systemic anti-tumor immune response and abscopal effect. Qu et al. [8]. show that histotripsy of flank tumors significantly reduced pulmonary metastases compared to controls (p<0.05) (Fig. 4). In addition, in a two-tumor model, histotripsy of one tumor inhibited the growth of contralateral untreated tumors [8,9]. These findings were hypothesized to be caused by the release of tumor-specific antigens from the histotripsy-produced acellular tumor debris and the immunological tumor death caused by histotripsy.
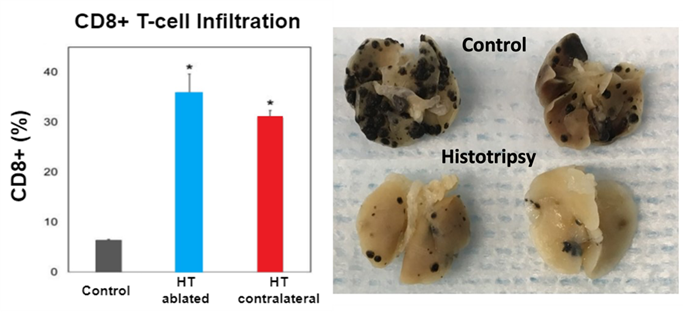
Fig. 4: (Left) In a two mouse tumor model, histotripsy-ablated (HT ablated) and contralateral non-ablated tumors (HT abscopal) resulted in comparable levels of intratumoral CD8+ T cell infiltration that is significantly higher than the untreated control tumors. (Right) Pulmonary metastases were reduced in mice treated with histotripsy compared to untreated controls.
References:
[1] Worlikar, T., Zhang, M., Ganguly, A., Hall, T. L., Shi, J., Zhao, L., Lee, F. T., Mendiratta- Lala, M., Cho, C. S. & Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers (Basel) 14, 1612, (2022). PMC8996987
[2] Worlikar, T., Mendiratta-Lala, M., Vlaisavljevich, E., Hubbard, R., Shi, J., Hall, T. L., Cho, C. S., Lee, F. T., Greve, J. & Xu, Z. Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model. BME Frontiers, (2020).
[3] Hall, T. L., Hempel, C. R., Wojno, K., Xu, Z., Cain, C. A. & Roberts, W. W. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 74, 932-937., (2009). PMC2757508
[4] Schade, G. R., Styn, N. R., Ives, K. A., Hall, T. L. & Roberts, W. W. Prostate histotripsy: evaluation of prostatic urethral treatment parameters in a canine model. BJU Int 113, 498- 503, (2014). PMC3944657
[5] Schade, G. R., Keller, J., Ives, K., Cheng, X., Rosol, T. J., Keller, E. & Roberts, W. W. Histotripsy Focal Ablation of Implanted Prostate Tumor in an ACE-1 Canine Cancer Model. The Journal of Urology 188, 1957-1964, (2012).
[6] Styn, N. R., Hall, T. L., Fowlkes, J. B., Cain, C. A. & Roberts, W. W. Histotripsy of renal implanted VX-2 tumor in a rabbit model: investigation of metastases. Urology 80, 724- 729, (2012).
[7] Hall, T. L., Kieran, K., Ives, K., Fowlkes, J. B., Cain, C. A. & Roberts, W. W. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. Journal of endourology / Endourological Society 21, 1159-1166, (2007).
[8] Qu, S., Worlikar, T., Felsted, A. E., Ganguly, A., Beems, M. V., Hubbard, R., Pepple, A. L., Kevelin, A. A., Garavaglia, H., Dib, J., Toma, M., Huang, H., Tsung, A., Xu, Z. & Cho, C. S. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 8, (2020). PMC7057529
[9] Pepple, A. L., Guy, J. L., McGinnis, R., Felsted, A. E., Song, B., Hubbard, R., Worlikar, T., Garavaglia, H., Dib, J., Chao, H., Boyle, N., Olszewski, M., Xu, Z., Ganguly, A. & Cho, C. S. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front Immunol 14, 1012799, (2023). PMC9900174
[10] Schuster, T. G., Wei, J. T., Hendlin, K., Jahnke, R. & Roberts, W. W. Histotripsy Treatment of Benign Prostatic Enlargement Using the Vortx Rx System: Initial Human Safety and Efficacy Outcomes. Urology, (2018).
[11] Vidal-Jove, J., Serres, X., Vlaisavljevich, E., Cannata, J., Duryea, A., Miller, R., Merino, X., Velat, M., Kam, Y., Bolduan, R., Amaral, J., Hall, T., Xu, Z., Lee, F. T., Jr. & Ziemlewicz, T. J. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. Int. J. Hyperthermia 39, 1115-1123, (2022).
[12] Vidal-Jove, J., Serres-Creixams, X., Ziemlewicz, T. J. & Cannata, J. M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans Ultrason Ferroelectr Freq Control 68, 3001-3005, (2021).
[13] Vlaisavljevich, E., Kim, Y., Allen, S., Owens, G., Pelletier, S., Cain, C., Ives, K. & Xu, Z. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med. Biol. 39, 1398-1409, (2013). 3709011
[14] Vlaisavljevich, E., Owens, G., Lundt, J., Teofilovic, D., Ives, K., Duryea, A., Bertolina, J., Welling, T. H. & Xu, Z. Non-Invasive Liver Ablation Using Histotripsy: Preclinical Safety Study in an In Vivo Porcine Model. Ultrasound Med. Biol. 43, 1237-1251, (2017).
[15] Kim, Y., Vlaisavljevich, E., Owens, G. E., Allen, S. P., Cain, C. A. & Xu, Z. In vivo transcostal histotripsy therapy without aberration correction. Phys. Med. Biol. 59, 2553- 2568, (2014).
