Congenital Heart Disease
Histotripsy has been investigated for treatment of hyperplastic left heart syndrome (HLHS), in which patients are born with an underdeveloped or even absence of left ventricle. As part of the reconstructive surgery, a flow channel needs to be created between left and right chambers of the heart via a perforation through the atrial septum in the neonatal HLHS patients [1]. Guided by ultrasound imaging, histotripsy was used to successfully create a perforation through the atrial septum in the in vivo open canine heart [2] and a perforation through the ventricular septum in the neonatal pig through an intact chest [3,4]. The short pulse duration (3 cycles at 750kHz-1MHz) at a pulse repetition frequency (PRF) of 1-20kHz and peak negative pressure of 11-15 MPa were shown to be efficient in creating perforations through the cardiac tissue [5].
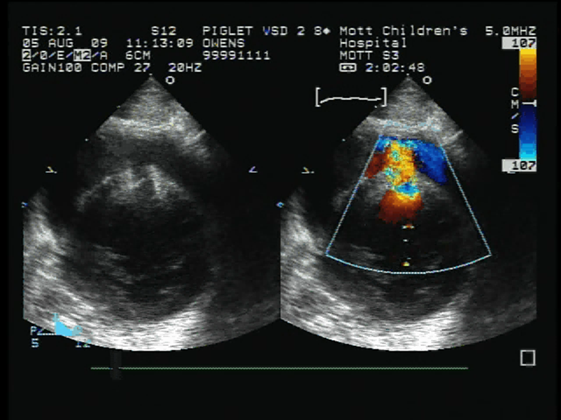
Fig. 1: Ultrasound B-mode image (left) and color Doppler image (right) show a flow channel was created through the ventricular septum in the in vivo neonatal porcine heart using histotripsy delivered through an intact chest.
Thrombosis
Thrombosis, blood clot formation, is a major cause of many cardiovascular diseases. Histotripsy has been shown to mechanically liquefy the blood clot into micron-sized debris to treat thrombosis [6-10]. Specifically, histotripsy has been investigated for the treatment of deep vein thrombosis, the formation of blood clots in the deep veins in extremities. We investigated the in vivo feasibility of the microtripsy thrombolysis in an in vivo porcine deep vein thrombosis model [8,11]. Microtripsy uses 1-cycle ultrasound pulses with only one high amplitude negative pressure phase to initiate cavitation when the negative pressure directly exceeds the intrinsic threshold of generating cavitation (26-30MPa in water-based tissue). Acute thrombi were formed in the left femoral veins of pigs (~35 kg) by occluding the vessel using two balloon catheters and infusing with thrombin. Guided by ultrasound imaging, microtripsy thrombolysis treatment was conducted in 14 pigs using 1 µs-long pulses at a PRF of 100Hz and a peak negative pressure of 30 MPa by a 1 MHz transducer. 10 pigs were euthanized on the same day (acute) and 4 at 2 weeks (subacute). To evaluate the vessel damage, 30-min free-flow treatment (no thrombus) in the right femoral vein was also conducted in 8 acute pigs. Blood flow was restored or significantly increased after treatment in 13 out of the 14 pigs confirmed by ultrasound color Doppler. The flow channels reopened by microtripsy had a diameter up to 64% of the vessel diameter (~6mm). The average treatment time was 16 minute per cm-long thrombus. Minor hemolysis was observed in both thrombolysis and free-flow treatments. Histology showed no vessel damage and only microscopic hemorrhage outside the veins for the free-flow treatments with nothing abnormal observed for the subacute treatments.
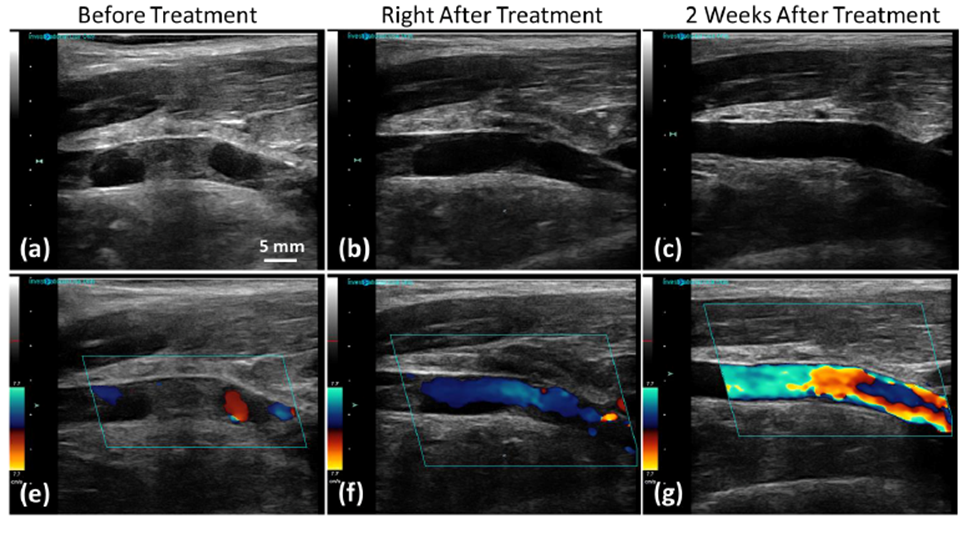
Fig. 2: Ultrasound B-mode (top) and color Doppler (bottom) images show completely occluded femoral vein before treatment in the in vivo porcine deep vein thrombosis model. The blood flow was restored through the femoral vein after histotripsy treatment, and the blood flow velocity further increased in two weeks after treatment.
References
[1] Rai, V., Gladki, M., Dudynska, M. & Skalski, J. Hypoplastic left heart syndrome [HLHS]: treatment options in present era. Indian J Thorac Cardiovasc Surg 35, 196-202, (2019). PMC7525540
[2] Xu, Z., Owens, G., Gordon, D., Cain, C. A. & Ludomirsky, A. Non-invasive Creation of an Atrial Septal Defect by Histotripsy in a Canine Model. Circulation 121, 742-749, (2010). PMC2834201
[3] Owens, G. E., Miller, R. M., Ensing, G., Ives, K., Gordon, D., Ludomirsky, A. & Xu, Z. Therapeutic ultrasound to non-invasively create intracardiac communications in an intact animal model. Catheter Cardiovasc Interv. 77, 580-588, (2011). PMC3010446
[4] Owens, G. E., Miller, R. M., Owens, S. T., Swanson, S. D., Ives, K., Ensing, G., Gordon, D. & Xu, Z. Intermediate-term effects of intracardiac communications created noninvasively by therapeutic ultrasound (histotripsy) in a porcine model. Pediatric cardiology 33, 83-89, (2012).
[5] Xu, Z., Ludomirsky, A., Eun, L. Y., Hall, T. L., Tran, B. C., Fowlkes, J. B. & Cain, C. A. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51, 726-736, (2004). PMC2669757
[6] Maxwell, A. D., Cain, C. A., Duryea, A. P., Yuan, L., Gurm, H. S. & Xu, Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy – histotripsy. Ultrasound Med. Biol. 35, 1982-1994, (2009). 2796469
[7] Maxwell, A. D., Owens, G., Gurm, H. S., Ives, K., Myers, D. D., Jr. & Xu, Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. Journal of vascular and interventional radiology 22, 369-377, (2011). 3053086
[8] Zhang, X., Macoskey, J. J., Ives, K., Owens, G. E., Gurm, H. S., Shi, J., Pizzuto, M., Cain, C. A. & Xu, Z. Non-Invasive Thrombolysis Using Microtripsy in a Porcine Deep Vein Thrombosis Model. Ultrasound Med Biol 43, 1378-1390, (2017). PMC5440202
[9] Zhang, X., Jin, L., Vlaisavljevich, E., Owens, G. E., Gurm, H. S., Cain, C. A. & Xu, Z. Noninvasive thrombolysis using microtripsy: a parameter study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 62, 2092-2105, (2015).
[10] Shi, A., Lundt, J., Deng, Z., Macoskey, J., Gurm, H., Owens, G., Zhang, X., Hall, T. L. & Xu, Z. Integrated Histotripsy and Bubble Coalescence Transducer for Thrombolysis. Ultrasound Med Biol 44, 2697-2709, (2018). PMC6215517
[11] Stocker, G. E., Shi, J., Ives, K., Maxwell, A. D., Dayton, P. A., Jiang, X., Xu, Z. & Owens, G. E. In Vivo Porcine Aged Deep Vein Thrombosis Model for Testing Ultrasound-based Thrombolysis Techniques. Ultrasound Med Biol 47, 3447-3457, (2021). PMC8578380
